News Release (Ordered Matter Science Research Center) Recently, Professor Wei-Qiang Liao and colleagues from the Ordered Matter Science Research Center at Nanchang University have achieved important progress in the field of molecular ferroelectric catalysis. Utilizing the H/F substitution strategy of Ferroelectrochemistry, they designed and synthesized a novel two-dimensional hybrid perovskite ferroelectric, [DFPD]₂CuCl₄, and for the first time applied it to catalyze the selective coupling reaction of alkynes. This catalytic system exhibits ultra-high activity and stability under mild conditions. The related research findings have been published in Chemical Science under the title "Organic-Inorganic Perovskite Ferroelectric Catalytic Selective Alkyne Coupling Under Ultrasound Sonication." Dr. Jun-Chao Qi from the International Institute of Ordered Matter Science is the first author of the paper.
Alkyne coupling reactions are one of the core reactions for constructing carbon-carbon bonds in organic synthesis, with their products, 1,3-diynes, widely used in materials chemistry, natural product synthesis, and pharmaceutical research. Traditional synthesis methods rely on precious metal catalysts or harsh reaction conditions (e.g., high temperature, excess base) (Figure 1, top left), suffering from limitations such as high cost, poor stability, and environmental unfriendliness. Ferroelectrics, as a class of multifunctional materials possessing spontaneous polarization, offer a new pathway for catalytic reactions through their polarization-induced internal electric fields and polarization changes under external fields, which can promote charge separation. Compared to commonly used inorganic ferroelectrics, molecular ferroelectrics exhibit better acoustic impedance matching with solvents, facilitating more efficient energy transfer. Furthermore, molecular ferroelectrics can be dissolved in common solvents, enabling regeneration and recycling, granting them significant advantages in ferroelectric catalysis. The team had previously achieved staged breakthroughs using molecular ferroelectrics in catalytic synthesis of quinoline derivatives (Nat. Commun. 2024, *15*, 6738), water splitting for hydrogen production (J. Am. Chem. Soc. 2025, *147*, 12635-12643), and difunctionalization of alkenes (Adv. Funct. Mater. 2025, *35*, 2502822). However, molecular ferroelectric catalysis is still in its infancy, and its catalytic potential in alkyne coupling remained unexplored.
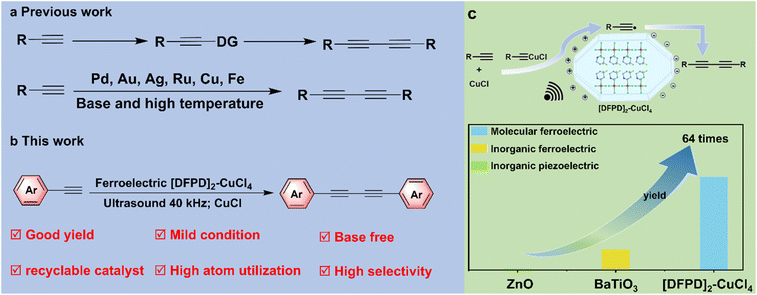
Figure 1. (Top left) Traditional synthetic routes to 1,3-diynes. (Bottom left) Molecular ferroelectric-catalyzed alkyne coupling strategy. (Right) Schematic illustration of the ferroelectric catalysis mechanism and a comparison of catalytic activity among the molecular ferroelectric [DFPD]₂CuCl₄, traditional inorganic ferroelectric BaTiO₃, and inorganic piezoelectric ZnO.
The team first utilized the hydrogen/fluorine substitution strategy to design and synthesize the novel 2D hybrid perovskite ferroelectric [4,4-difluoropiperidinium]₂CuCl₄ ([DFPD]₂CuCl₄), which possesses both a high Curie temperature (398 K) and room-temperature ferroelectricity. Subsequently, it was applied to catalyze alkyne coupling reactions. Under conditions of 40 kHz ultrasound at room temperature and atmospheric pressure, and without the need for additional base reagents (Figure 1, bottom left), this system achieved highly selective coupling of various terminal alkynes. The selectivity for the target 1,3-diyne products reached up to 99%, with yields up to 88%. Compared to traditional inorganic ferroelectrics (e.g., barium titanate, BaTiO₃) and piezoelectric materials (e.g., zinc oxide, ZnO), the catalytic activity of [DFPD]₂CuCl₄ was significantly enhanced, being approximately 4.5 times that of BaTiO₃ and 64 times that of ZnO (Figure 1, right). Its core advantages lie in the good acoustic impedance matching between the molecular ferroelectric and the solvent, leading to high energy transfer efficiency, and its excellent cycling stability. After 10 catalytic cycles, its crystal structure, ferroelectric properties, and catalytic activity remained unchanged. Furthermore, the reaction products could be directly purified via crystallization due to their high yields. Some products also exhibited structural phase transition characteristics, providing new ideas for the development of functional materials. This work represents the first application of a molecular ferroelectric in catalyzing alkyne coupling, realizing an efficient and sustainable novel catalytic system, which holds significant importance for advancing the catalytic applications of molecular ferroelectrics.
Link to original article: https://pubs.rsc.org/en/Content/ArticleLanding/2025/SC/D5SC07784B